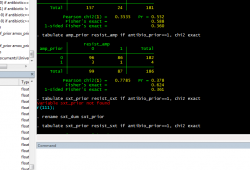
The day-to-day routine of epidemiology is not always thrilling. Although fieldwork and data collection are fun, once you've collected the data you have to analyze it - and before you can analyze it, you have to clean it. Before I started my internship, data cleaning was a mystery to me, but after spending 3 weeks cleaning a rather large data set I've realized that it's mostly a tedious process - going through each individual observation (there can be anywhere from a few hundred to over ten thousand) and making sure that all of the variables make sense. However tedious it is, it's one of the most important steps in the analysis. Computers are fundamentally stupid and only do (exactly) what you tell them - if that wasn't enough, they have a hard time figuring out what letters are, so a major part of data cleaning and entry is devising codes to convert your data into a series of numbers. It's not exactly the most glamorous part of research, but it needs to be done before you can start asking the questions you set out to answer. The upside of data cleaning is that I spend most of my day looking at screens like the one below: It appears that I'm rapidly approaching the end of the data cleaning and finishing up some of my analyses, and I'll be off to Liwonde for the next 2-3 weeks to start collecting data from the district hospital there. Thankfully, I'll be able to put the computer away for a few weeks and start to learn how the data that make up our datasets get collected.
0 Comments
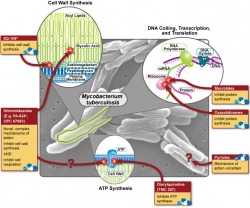
The Office of Public Health Practice hosted their annual symposium on Wednesday, and the theme was "Can the World be TB Free"? I only had a chance to attend one of the talks (by Dr. Joseph McCormick), which dealt with the rising problem of multi-drug resistant tuberculosis (MDR-TB) and how our treatment strategies of TB may have helped this new disease to emerge. In what is sadly a familiar story for many bacterial diseases, the discovery of streptomycin (the first antibiotic that was effective against TB) was hailed as the first step in the elimination of the disease, but as time as passed the drug has become less and less effective, forcing us to search for new treatments. In the wake of the HIV/AIDS epidemic, TB has exploded and the growing problem of antibiotic resistance makes treating these people very difficult. So why does drug resistance happen, and how is it our fault? There are about 10 million (or 10^7 if you're feeling scientific) individual tuberculosis bacteria living in each cavity. About one bacteria out of every 10-100 million will randomly develop a mutation that confers resistance to any one of the two major first line drugs, rifampin and isonazid. These mutations are quite rare, but given that bacteria are nothing if not effective reproducers, it is safe to assume that approximately one bacterium per cavity is resistant to rifampin, and that another is resistant to isonazid. This situation may not sound all that bad, but consider what would happen if the patient were to be treated with rifampin alone - every single bacterium would die except for the one that had developed resistance. This bacterium is now presented with perfect growth conditions - no competition and lots of food - so it begins to multiply, and after a few days have passed, 10 million bacteria live in the cavity again - but this time all of them are resistant to rifampin. Given the large number of bacteria involved, it's now reasonable to expect that one of these resistant bacteria will then develop a resistance to isonazid, and following another single-drug treatment cycle, MDR-TB is born.
Our bodies are not our own - our intestinal tracts are colonized by an amazing variety of bacterial species - and we're just now realizing how dramatic their effect on our life truly is. A quick search shows that researchers are investigating the role of bacteria in processes as diverse as obesity, Crohn's Disease, immune suppression, blood clotting, and nutritional disorders. However, a recent talk by Dr. Vincent Denef of the University of California, Berkeley, suggests that we may be going about these investigations in the wrong way.His argument centers on the fact that two things that look alike may not act alike - and that these small differences can drastically change the ecology of entire systems. Until the advent of genomic sequencing and PCR, the only way to discover if a bacteria lived somewhere was to try to isolate it and regrow it in your lab. Unfortunately, only about 0.01% of bacteria can grow under laboratory conditions, so researchers switched to using DNA bar codes - they found a certain DNA sequence served that as a unique "fingerprint" for different species. Or so they thought.
Dr. Denef has shown that even bacteria with the same fingerprint can have slightly different genomic profiles, and that these different profiles can change the bacterial composition. To put the idea of small genetic differences leading to large changes in phenotype and ecology, consider that the genomes of humans and chimpanzees are 99.9% similar - but that differing 0.01% makes a world of difference. Do these types of difference matter in bacteria? The answer is a resounding yes. One all-too-common example of small differences within the same species is antibiotic resistance. Not all strains of Staphylococcus aureus (the bug that causes staph infections) are harmful, and most people's skin is permanently colonized with these bacteria to no ill effect. But if you get infected with MRSA - the drug resistant strain - it will lead to a medical emergency. However, if you compared the normal strain to the resistant stain using the same techniques we use to fingerprint bacteria, they'd come up as the same species. Antibiotic resistance is the best example in bacteria that affect humans, and right now we just don't know how common these differences are in the bacteria living in our gut. They could be common, and they could have drastic effects (imagine if these kinds of differences caused irritable bowel syndrome!) or they could just end up being a scientific curiosity. But we won't know until we look. One of the things that I really like about public health is it's broad scope: sometimes it seems that pretty much everything has some impact on health. While these influences may be non-intuitive, their effect on health can be huge. Climate change is a good example. Whatever your thoughts on the subject, global temperatures and weather patterns are changing at an especially rapid pace, and this change is likely to cause a lot of problems. The Center for Global Health here at the University of Michigan hosted a nice talk today by Howard Hu about some of climate change's possible ramifications. His research focuses on environmental health, specifically air quality, so the presentation was a bit heavy on the negative effects of fine (2.5 micron!) particles produced by burning fossil fuels (it's bad, when the atmospheric concentration gets too high, risk of heart disease increases significantly). However, he also talked about how climate change will change a lot of the factors that we take for granted - especially that certain "tropical" diseases, like Dengue Fever and Malaria will stay in the tropics.
The diseases that he focused were those that are spread by mosquitoes. If the earth warms, we expect that there will be many more warm low-lying swampy areas, and these regions provide mosquitoes with an ideal habitat. In Africa, malaria is the single largest cause of death in children under 5. More than 110 million people are currently at risk of acquiring malaria, and based on some conservative projections (it should be stressed that "projection" does not mean "this is guaranteed to happen") up to 500 million people could be at risk in the near future. Perhaps most frighteningly (for us in the US anyways) is that the American South and Northern Europe, especially along the Baltic coast, are predicted as likely habitats. It's not just patterns of malaria incidence that will be changed; a very nice recent study by Danovaro et al. in PLoS ONE determined that changing patterns in the distribution of marine snow in the Mediterranean could act as a vector for spreading bacterial pathogens. Who knew that public health might lead you to study microscopic marine algae? It's been known for quite some time that climate impacts health - the environment is one of the three key features in the classical "epidemiologic triad", but the recognition that changing climate patterns (which also occurs naturally) can have far-reaching consequences on health is new and has opened up a whole new field (literally) and possibilities for transdisciplinary research.  Apologies for not updating sooner; the readjustment process has been chaotic (putting it mildly) and the blog here fell off my radar screen for a while. My last week in Germany was also rather busy, between some traveling in the region (post on Eisenach coming soon) and the annual MPI soccer tournament. I'm happy to say that our team (the Rocket Cows) came in 5th overall (out of 12), which isn't too bad for never having practiced. My arrival in the US kept the general level of activity high - a few days after arriving we left for a family vacation to New York City (my first time there), where we had a great time. Pictures for the interested. NYC was a shock - even the largest cities I'd seen in Europe couldn't compare to the overall activity level of NYC, and the massive American flag presence (it seemed like every street corner...) made sure that I knew where I was.  I've also been getting my fair share of US (well, southern US) "culture" - lots of BBQ and grilling, as well as going shooting and "creek walking". Creek walking was new to me but it's becoming one of my favorite activities. It consists of finding a creek, and then seeing how far up the creek you can travel without getting wet, with crossing from one to the other as you see fit to add a challenge. The creeks here in the Smoky Mountains are riddled with boulders, so moving up the creek requires a combination of climbing, jumping, and creative movement skills (as seen by my friend Cisco's boulder hopping in the picture). Of course, you end up getting wet (falling in, misjudging a jump, slipping on moss...), and then walking back through the cold water on a warm day just adds to the fun. After this pleasant interlude, I'll be heading off to the University of Michigan early tomorrow morning, which will require me to re-enter academic mode and see how well I actually tolerate cold environments (I'm hoping well, but lake-effect snow scares me). Starting my MPH is exciting, and I'm looking forward to the new curriculum and opportunities. In a somewhat more intellectual vein, I recently had a paper published - it's not accessible online yet (unless you're luck enough to have a subscription through a university) but you can find the abstract here. Needless to say, I'm very excited and will share the paper here if possible (i.e. if it ever becomes open-access).
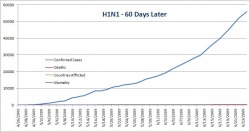
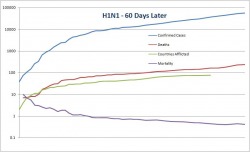
Forget cockroaches. Bacteria will be the only organisms left if we manage to launch a warhead too many or if any doomsday scenarios actually occur. It's a good thing - without bacteria we wouldn't be alive. Bacteria control nutrient cycles and help us make cheese, but they can also kill us (it should be noted that pathogenic bacteria make up a minuscule portion of the known bacteria). Their astounding diversity has allowed them to become the earth's dominant life form. New research from Mitchell et al. (Nature, July 2009) seems to indicate that higher animals (i.e., those of us lucky enough to have a central nervous system) aren't the only ones able to prepare for the future based on current environmental conditions - bacteria (E. coli) and yeast (S. cerevisiae) can do it too. While it seems odd, the basic principle has been recognized for years. Indeed, the lac operon in E. coli activates the genes needed to digest the sugar lactose, but the lac operon is activated only by lactose. This mechanism makes good sense from an evolutionary perspective - proteins are expensive to produce, and if the cell does not need them (i.e. there is no lactose to digest) then producing them is wasteful. While direct links like this one are easy to uncover, more subtle ones have remained a mystery - until now. Sixty days ago, a novel flu strain from Mexican pigs brought the world to a minor panic. As the data started to come in, it looked as if the strain was a serious illness in young, healthy victims - the same pattern seen with the 1918 outbreak. The initial mortality rate was just under 10%, a shockingly high figure, but after the first two weeks the rate had dropped down to 1%, mostly a result of more thorough testing of those with flu-like symptoms. In that same time frame, pig farmers and the pork lobby fought to change the name from "swine flu" to "Influenza A (H1N1)", a change which the WHO adopted. While it may seem a bit silly, the name change helped to reduce panic - think about how many warnings you got to avoid eating pork (which is just silly - cooking the pork would kill the virus, and even if you ate truckloads of infected raw pork, which I don't recommend, the viral particles are not going to survive the passage through the intestinal track. Within three weeks, global coverage of H1N1 dropped to a trickle. If the virus is mentioned today, it is usually in connection with the first death in a new country. Now that we've got the background and the dangers covered, how severe has H1N1 actually been? The WHO has been kind enough to publish all of their data, including confirmed cases and mortality broken down by country (here). I used these data to make up a graph showing confirmed cases, mortality, number of countries afflicted, and mortality rate over time. As you can see below, when you use a standard y-axis the mortality is so low that you can't even see it when compared to the overall number of cases (for the record, the mortality rate is about 0.4% - slightly higher than, but on the same order of magnitude as seasonal flu) Since using a normal axis wasn't very helpful (it's hard to extract data from a graph that you can't read) I also made a log scale graph. It looks slightly scary, but please, pay attention to the numbers on the y-axis and note that the values increase logarithmically. On this graph it's fairly easy to see that while both the confirmed cases and mortality are still climbing, the overall mortality rate is fairly constant. I attempted some curve-fitting to these lines (power regressions) but the r-value was too low for the trendlines to mean anything - so we need to wait on more data before we can make any real predictions. In this case the x-axis has the same scale as the above graph. If anyone wants the original Excel file, let me know and I'll send it to you. To conclude, I'd like to give a short caveat about this data set. It's likely that it underestimates the total number of confirmed cases (how many people with flu-like symptoms have not been tested for H1N1 around the world?), while the total number of deaths is likely to be fairly accurate - if someone dies from flu-like respiratory symptoms their blood will probably be tested. Given these two assumptions (and I stress that they are assumptions), the mortality rate may be slightly lower than the data suggest, which is certainly a Good Thing. Although we now have much more data about this pandemic virus, which appears to be much less deadly than originally feared, there's still a lot of factors that are outside of our control. What happens if the virus mutates or recombines? What effect will it have on the immuno-suppressed (AIDS victims and people with chronic parasite infections, both of which are most common in the developing world)? These are all good questions, and although we can use the data (yay epidemiology!) to help make predictions, until flu season starts again we can't say anything for certain.
Bacteria are everywhere. It's estimated that there are over 100 trillion (with a t) individual bacteria living in the average humans digestive tract. While it sounds fairly disgusting (unless, of course, you're a microbiologist), their presence is actually a Good Thing - these bacteria help us to break down and digest foods that we would normally not be able to eat on our own. However, sometimes this symbiosis has some unintended consequences, as this wonderful paper in PLoS ONE shows. With the ever-increasing use of medial imaging technology, such as CT scanning and MRI, it is now fairly common for tumors to be detected incidentally - that is, before they start to cause pain or produce symptoms on their own. While this incidental detection is a Good Thing (certainly better then only discovering a tumor after it has metastasized), in the case of small renal masses (tumors <4cm) - which can be either renal cell carcinomas (kidney cancer) or benign - the best way to treat these tumors is still a matter of (very) heated discussion. Standard procedure used to be a complete removal of the kidney - after all, we each have two, and if you remove the kidney you're guaranteed not to leave any positive margins. However, some recent studies have shown that mortality increases after a kidney is removed - even from factors not related to normal renal function. Consequently, there has been a trend towards removing part of the kidney in order to preserve renal function. Surgical intervention is an inherently risky undertaking, and despite what medical TV shows would have you believe, the average patient is not a young, attractive, generally healthy person - they are often elderly with many other comorbidities, such as heart disease. In these patients, the surgery can often be more traumatic than the disease itself. |
Somewhere Else
 RSS Feed
RSS Feed